Technology Platform Light Microscopy
The Technology Platform Light Microscopy (TPLM) operates advanced light microscopes and image analysis resources and develops new technologies for light microscopy since 2022.
The TPLM provides access to light microscopes at the Centre for Structural Systems Biology (CSSB) in the Advanced Light and Fluorescence Microscopy (ALFM) Core Facility and at the Department of Biology with the Light Microscopy Platform at the Institute of Plant Science and Microbiology (IPM) and the Institute of Cell and Systems Biology of Animals (IZS).
Mission
The mission of the Technology Platform Light Microscopy (TPLM) is to enable researchers to use the full spectrum of advanced light microscopy technologies for their research projects. To fulfill this mission, the TPLM has the following tasks:
- Infrastructure: The TPLM provides access to state-of-the-art light microscopy infrastructure and image analysis resources and regularly renews and expands its equipment.
- Training and Support: The TPLM assists researchers in selecting the best imaging and image analysis methods for their studies, trains researchers in the use of TPLM equipment, assists researchers in solving problems that arise during ongoing projects, and conducts collaborative projects with research groups.
- Method Development: The TPLM develops new methods and technologies for light microscopy that are of use for researchers on campus.
- Teaching: The TPLM offers courses on the basics of light microscopy and advanced light microscopy techniques.
What support is offered by the TPLM?
The TPLM offers several types of support according to the need of the researchers:
- Independent use of equipment after training
- Accompanying support of imaging projects
- Joint development and implementation of projects in the field of light microscopy.
We also support researchers in image processing and quantitative analysis of light microscopy data.
Contact and access to the equipment
The TPLM currently provides access to light microscopes in the ALFM Facility at CSSB Hamburg. The ALFM Facility is also accessible to external users.
The technology Platform Light Microscopy team supports you in planning and performing light microscopy experiments and in analyzing light microscopy image data. Please contact the platform coordinator Roland Thünauer for further information.
Locations
Centre for Structural Systems Biology (CSSB)
c/o Deutsches Elektronen-Synchrotron DESY
Notkestraße 85, Building 15
22607 Hamburg
Light Microscopy Platform at the Deparment of Biology
Institute of Plant Sciences and Microbiology (IPM)
Ohnhorststr. 18
22609 Hamburg
Institute of Cell and Systems Biology of Animals (IZS)
Martin-Luther-King-Platz 3
20146 Hamburg
How to use the devices?
For new users, a meeting will be arranged to discuss how the TPLM can support you and how to access equipment at TPLM.
Please contact Roland Thünauer to arrange a meeting or to get more information.
How can devices be booked?
At the ALFM Facility at CSSB Hamburg:
Devices at the ALFM Facility can be booked through the PPMS booking calendar. Access to the booking system will be granted after a training and signing of the usage conditions (PDF). Further information can be found at the ALFM Webpage
At the Department of Biology:
For information and questions about the Biology Department, please contact Roland Thünauer.
Projects
The TPLM is open to collaborative projects with a particular focus on method development for light microscopy.
Current projects
CUI: AIM projekt cryoLM
In this project, new illumination schemes for light cryo-microscopy (cryoLM) are developed.
Cooperation partners:
Kay Grünewald, Franz Kärtner, Kartik Ayyer, Rainer Kaufmann und Felix Lehmkühler
Publications
The TPLM is open to collaborate with researchers on scientific projects with a particular focus on method development for light microscopy.
How can you acknowledge TPLM in publications?
If you are using equipment or support from the Technology Platform Light Microscopy (TPLM), we ask you to indicate this in your publication.
The text below can be used as template:
We thank the Technology Platform Light Microscopy (TPLM) at Universität Hamburg (UHH), in particular XXX, for support with light microscopy image acquisition and analysis.
Publications with contributions from TPLM
2023
A TriPPPro-Nucleotide Reporter with Optimized Cell-Permeable Dyes for Metabolic Labeling of Cellular and Viral DNA in Living Cells.
Sterrenberg VT, Stalling D, Knaack JIH, Soh TK, Bosse JB, Meier C.
Angew Chem Int Ed Engl. 2023; 62(38):e202308271. doi: 10.1002/anie.202308271.
PMID: 37435767
Kupffer cell-like syncytia replenish resident macrophage function in the fibrotic liver.
Peiseler M, Araujo David B, Zindel J, Surewaard BGJ, Lee WY, Heymann F, Nusse Y, Castanheira FVS, Shim R, Guillot A, Bruneau A, Atif J, Perciani C, Ohland C, Ganguli Mukherjee P, Niehrs A, Thuenauer R, Altfeld M, Amrein M, Liu Z, Gordon PMK, McCoy K, Deniset J, MacParland S, Ginhoux F, Tacke F, Kubes P.
Science. 2023; 381(6662):eabq5202. doi: 10.1126/science.abq5202.
PMID: 37676943
Real-time monitoring of cell surface protein arrival with split luciferases.
Fischer AAM, Schatz L, Baaske J, Römer W, Weber W, Thuenauer R.
Traffic. 2023; 24(10):453-462. doi: 10.1111/tra.12908.
PMID: 37403269
Global analysis of putative phospholipases in Plasmodium falciparum reveals an essential role of the phosphoinositide-specific phospholipase C in parasite maturation.
Burda PC, Ramaprasad A, Bielfeld S, Pietsch E, Woitalla A, Söhnchen C, Singh MN, Strauss J, Sait A, Collinson LM, Schwudke D, Blackman MJ, Gilberger TW.
mBio. 2023; 14(4):e0141323. doi: 10.1128/mbio.01413-23.
PMID: 37489900
Comparative proteomics of vesicles essential for the egress of Plasmodium falciparum gametocytes from red blood cells.
Sassmannshausen J, Bennink S, Distler U, Küchenhoff J, Minns AM, Lindner SE, Burda PC, Tenzer S, Gilberger TW, Pradel G.
Mol Microbiol. 2023. doi: 10.1111/mmi.15125. Online ahead of print.
PMID: 37492994
A validated protocol to UV-inactivate SARS-CoV-2 and herpesvirus-infected cells.
Soh TK, Pfefferle S, Wurr S, von Possel R, Oestereich L, Rieger T, Uetrecht C, Rosenthal M, Bosse JB.
PLoS One. 2023; 18(5):e0274065. doi: 10.1371/journal.pone.0274065.
PMID: 37163509
Non-functional ubiquitin C-terminal hydrolase L1 drives podocyte injury through impairing proteasomes in autoimmune glomerulonephritis.
Reichelt J, Sachs W, Frömbling S, Fehlert J, Studencka-Turski M, Betz A, Loreth D, Blume L, Witt S, Pohl S, Brand J, Czesla M, Knop J, Florea BI, Zielinski S, Sachs M, Hoxha E, Hermans-Borgmeyer I, Zahner G, Wiech T, Krüger E, Meyer-Schwesinger C.
Nat Commun. 2023; 14(1):2114. doi: 10.1038/s41467-023-37836-8.
PMID: 37055432
CXCR5+PD-1++ CD4+ T cells colonize infant intestines early in life and promote B cell maturation.
Jordan-Paiz A, Martrus G, Steinert FL, Kaufmann M, Sagebiel AF, Schreurs RRCE, Rechtien A, Baumdick ME, Jung JM, Möller KJ, Wegner L, Grüttner C, Richert L, Thünauer R, Schroeder-Schwarz J, van Goudoever JB, Geijtenbeek TBH, Altfeld M, Pals ST, Perez D, Klarenbeek PL, Tomuschat C, Sauter G, Königs I, Schumacher U, Friese MA, Melling N, Reinshagen K, Bunders MJ.
Cell Mol Immunol. 2023; 20(2):201-213. doi: 10.1038/s41423-022-00944-4.
PMID: 36600048
Slow integrin-dependent migration organizes networks of tissue-resident mast cells.
Kaltenbach L, Martzloff P, Bambach SK, Aizarani N, Mihlan M, Gavrilov A, Glaser KM, Stecher M, Thünauer R, Thiriot A, Heger K, Kierdorf K, Wienert S, von Andrian UH, Schmidt-Supprian M, Nerlov C, Klauschen F, Roers A, Bajénoff M, Grün D, Lämmermann T.
Nat Immunol. 2023; 24(6):915-924. doi: 10.1038/s41590-023-01493-2.
PMID: 37081147
A Microtubule-Associated Protein Is Essential for Malaria Parasite Transmission.
Wichers-Misterek JS, Binder AM, Mesén-Ramírez P, Dorner LP, Safavi S, Fuchs G, Lenz TL, Bachmann A, Wilson D, Frischknecht F, Gilberger TW.
mBio. 2023; 14(1):e0331822. doi: 10.1128/mbio.03318-22.
PMID: 36625655
Adsorption and Inactivation of SARS-CoV-2 on the Surface of Anatase TiO2(101).
Kohantorabi M, Wagstaffe M, Creutzburg M, Ugolotti A, Kulkarni S, Jeromin A, Krekeler T, Feuerherd M, Herrmann A, Ebert G, Protzer U, Guédez G, Löw C, Thuenauer R, Schlueter C, Gloskovskii A, Keller TF, Di Valentin C, Stierle A, Noei H.
ACS Appl Mater Interfaces. 2023 doi: 10.1021/acsami.2c22078.
PMID: 36723177
Formation kinetics and physicochemical properties of mesoscopic Alpha-Synuclein assemblies modulated by sodium chloride and a distinct pulsed electric field.
Wang M, Thuenauer R, Schubert R, Gevorgyan S, Lorenzen K, Brognaro H, Betzel C.
Soft Matter. 2023 doi: 10.1039/d2sm01615j.
PMID: 36723049
Dimeric Lectin Chimeras as Novel Candidates for Gb3-Mediated Transcytotic Drug Delivery through Cellular Barriers.
Xu M, Antonova M, Salavei P, Illek K, Meléndez AV, Omidvar R, Thuenauer R, Makshakova O, Römer W.
Pharmaceutics. 2023 15(1):225. doi: 10.3390/pharmaceutics15010225.
PMID: 36678854
2022
Protocol for live-cell fluorescence-guided cryoFIB-milling and electron cryo-tomography of virus-infected cells.
Franken LE, Rosch R, Laugks U, Grünewald K.
STAR Protoc. 2022 3(4):101696. doi: 10.1016/j.xpro.2022.101696.
PMID: 36149798
MARVEL domain containing CMTM4 affects CXCR4 trafficking.
Bona A, Seifert M, Thünauer R, Zodel K, Frew IJ, Römer W, Walz G, Yakulov TA.
Mol Biol Cell. 2022 33(13):ar116. doi: 10.1091/mbc.E22-05-0152.
PMID: 36044337
Functional inactivation of Plasmodium falciparum glycogen synthase kinase GSK3 modulates erythrocyte invasion and blocks gametocyte maturation.
Alder A, Wilcke L, Pietsch E, von Thien H, Pazicky S, Löw C, Mesen-Ramirez P, Bachmann A, Burda PC, Kunick C, Sondermann H, Wilson D, Gilberger TW.
J Biol Chem. 2022 298(9):102360. doi: 10.1016/j.jbc.2022.102360.
PMID: 35961464
Nipah Virus Infection Generates Ordered Structures in Cellulo.
Vázquez CA, Widerspick L, Thuenauer R, Schneider C, Reimer R, Neira P, Olal C, Heung M, Niemetz L, Lawrence P, Kucinskaite-Kodze I, Redecke L, Escudero-Pérez B.
Viruses. 2022 14(7):1523. doi: 10.3390/v14071523.
PMID: 35891503
The Lectin LecB Induces Patches with Basolateral Characteristics at the Apical Membrane to Promote Pseudomonas aeruginosa Host Cell Invasion.
Thuenauer R, Kühn K, Guo Y, Kotsis F, Xu M, Trefzer A, Altmann S, Wehrum S, Heshmatpour N, Faust B, Landi A, Diedrich B, Dengjel J, Kuehn EW, Imberty A, Römer W.
mBio. 2022 13(3):e0081922. doi: 10.1128/mbio.00819-22.
PMID: 35491830
Human cytomegalovirus forms phase-separated compartments at viral genomes to facilitate viral replication.
Caragliano E, Bonazza S, Frascaroli G, Tang J, Soh TK, Grünewald K, Bosse JB, Brune W.
Cell Rep. 2022 38(10):110469. doi: 10.1016/j.celrep.2022.110469.
PMID: 35263605
Intermittent bulk release of human cytomegalovirus.
Flomm FJ, Soh TK, Schneider C, Wedemann L, Britt HM, Thalassinos K, Pfitzner S, Reimer R, Grünewald K, Bosse JB.
PLoS Pathog. 2022 18(8):e1010575. doi: 10.1371/journal.ppat.1010575.
PMID: 35925870
A non-reactive natural product precursor of the duocarmycin family has potent and selective antimalarial activity.
Alder A, Struck NS, Xu M, Johnson JW, Wang W, Pallant D, Cook MA, Rambow J, Lemcke S, Gilberger TW, Wright GD.
Cell Chem Biol. 2022 29(5):840-853.e6. doi: 10.1016/j.chembiol.2021.10.005.
PMID: 34710358
Available equipment
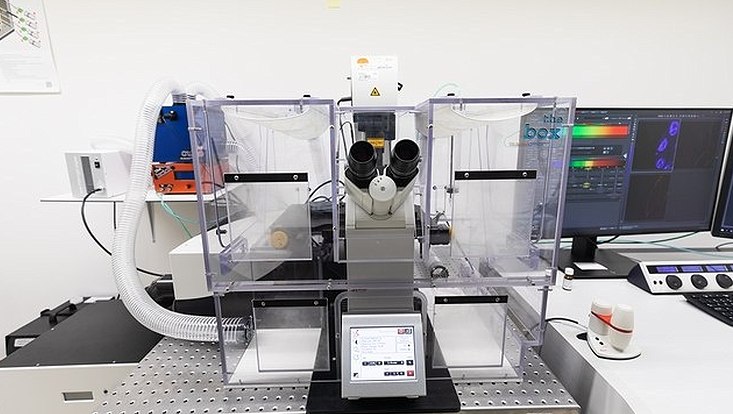
Photo: UHH/Feuerböther
Analysis computer
Description: The analysis computer has 768 GB of memory and two NVIDIA RTX A6000 graphics cards for processing and analyzing large amounts of image data. FIJI, Imaris, Arivis and Matlab image analysis software are available on the computer.
Main applications: Image processing and quantitative image analysis of microscopy data.

Photo: UHH/Feuerböther
SP8 confocal microscope with FALCON FLIM unit
Description: This inverted point-scanning confocal microscope from Leica is equipped with a 405 nm laser, a white light laser and 4 hybrid detectors. The microscope has an incubation unit for live cell imaging and a FALCON FLIM unit. The microscope is programmable via the Navigator module of the Leica LASX software. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Confocal microscopy, FLIM, FRET, live cell microscopy.

Photo: UHH/Feuerböther
cryoCLEM widefield microscope
Description: This upright widefield microscope from Leica is equipped with a cryo-stage for imaging vitrified specimens on electron microscopy grids under constant cooling with liquid nitrogen. A mercury metal halide burner (EL6000) is available as the fluorescence excitation light source. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Light cryo-microscopy, widefield microscopy, correlative light- and electron-cryo-microscopy.

Photo: UHH/Feuerböther
cryoCLEM Stellaris 8 confocal microscope
Description: This upright confocal microscope from Leica is equipped with a cryo-stage for imaging vitrified specimens on electron microscopy grids under constant cooling with liquid nitrogen. Available fluorescence excitation light sources include a 405 nm pulsed laser, a white light pulsed laser, and 488 nm, 561 nm, and 633 nm cw lasers. The microscope is equipped with four hybrid detectors and has a FLIM unit. In addition, the microscope can be operated in widefield mode and has LED a fluorescence widefield excitation light source. The microscope is programmable via the Navigator module of the Leica LASX software. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Light cryo-microscopy, confocal microscopy, FLIM, FRET, widefield microscopy, correlative light- and electron-cryo-microscopy.
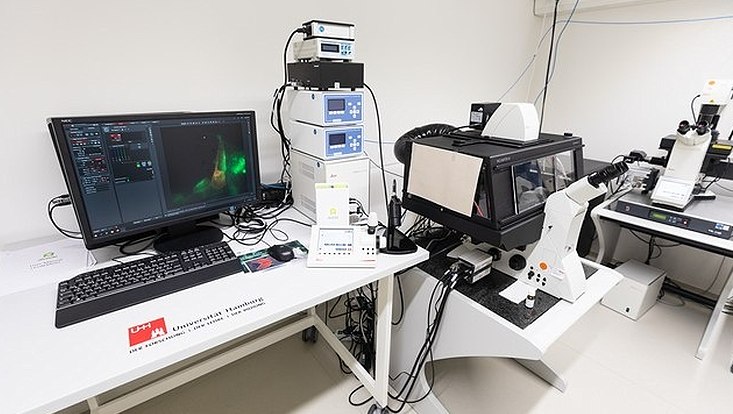
Photo: UHH/Feuerböther
DMi8 inverted widefield microscope for live cell imaging and with Primo Alveole micropatterning unit
Description: This inverted widefield microscope from Leica is equipped with an incubation unit for live cell measurements and an LED fluorescence excitation light source. In addition, a Primo Alveole micropatterning unit is attached. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Live cell microscopy, widefield microscopy, phase contrast microscopy, DIC, micropatterning.

Photo: UHH/Feuerböther
DMi8 inverted widefield microscope for live cell imaging and THUNDER Software
Description: This inverted widefield microscope from Leica is equipped with an incubation unit for live cell measurements, an LED fluorescence excitation light source, and THUNDER software for computational clearing. The microscope is programmable for complex tasks, such as scanning multiwell plates. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Live cell microscopy, widefield microscopy, phase contrast microscopy, DIC, scanning of multiwell plates.

Photo: UHH/Feuerböther
DM6 B upright widefield microscope #1
Description: This upright widefield microscope from Leica is equipped with a fluorescence excitation light source. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Widefield microscopy, phase contrast microscopy, DIC, histology.

Photo: UHH/Feuerböther
DM6 B upright widefield microscope #2
Description: This upright widefield microscope from Leica is equipped with a fluorescence excitation light source and is fully motorized. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Widefield microscopy, phase contrast microscopy, DIC, histology.

Photo: UHH/Feuerböther
DM6 B upright widefield microscope #3
Description: This upright widefield microscope from Leica is equipped with a fluorescence excitation light source. The microscope operated in the BSL2 lab of Tim Gilberger at CSSB Hamburg.
Main applications: Widefield microscopy, phase contrast microscopy, DIC, histology.

Photo: UHH/Feuerböther
SR GSD 3D Super-resolution microscope
Description: This inverted microscope from Leica is equipped for Single Molecule Localization Microscopy (SMLM) super-resolution microscopy and a special stage which is directly coupled to the objective. Fluorescence excitation can be performed in widefield mode or in Total Internal Reflection Fluorescence (TIRF) mode. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Super-resolution microscopy, TIRF, STORM, DNA-PAINT.

Photo: UHH/Feuerböther
Spinning-Disc and SIM microscope with FRAP unit
Description: This inverted microscope from Nikon has multiple modes in which it can be operated. There is a Yokogawa CSU W1 spinning disc unit with an Andor iXon EMCCD camera and a Nikon E-SIM unit for Structured Illumination Microscopy (SIM). In addition, the microscope is equipped with a Fluorescence Recovery after Photobleaching (FRAP) unit from Rapp Optoelectronics. An incubation unit for live cell measurements is available. The microscope is fully programmable via the JOBS module in Nikon NIS Elements software. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Spinning disc confocal microscopy, SIM, FRAP, photoactivation, live cell microscopy, scanning of multiwell plates.

Photo: UHH/Feuerböther
LSM880 Airyscan confocal microscope
Description: This inverted point-scanning confocal microscope from Zeiss has an Airyscan detector, which can be used to achieve an approximately 1.8-fold increase in resolution over normal confocal microscopes, and a spectral Imaging detector. It is equipped with an incubation unit for live cell measurements. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Confocal microscopy, live cell microscopy, airyscan microscopy.

Photo: UHH/Feuerböther
Elyra PS.1 Super-resolution microscope
Description: This inverted super-resolution microscope from Zeiss can be used for Single Molecule Localization Microscopy (SMLM) microscopy and Structured Illumination Microscopy (SIM). Fluorescence excitation can be performed in widefield mode or in Total Internal Reflection Fluorescence (TIRF) mode. It is equipped with an incubation chamber for live cell measurements. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Super-resolution microscopy, TIRF, STORM, PALM, DNA-PAINT, SIM, wide-field microscopy.

Photo: UHH/Feuerböther
Lattice light sheet microscope
Description: This lattice light sheet microscope from Zeiss is suitable for fast volumetric imaging of living cells with particularly low phototoxicity and simultaneously high local resolution. It features two Hamamatsu sCMOS cameras. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Lattice light sheet microscopy, live cell microscopy.

Photo: UHH/Feuerböther
Cell culture laminar flow work bench and incubators
Description: The cell culture laminar flow work bench and two incubators are fully equipped to allow ALFM Facility users to prepare and pre-incubate their live cell samples before microscopy. The cell culture workbench and the incubators are located in a biological safety level 2 (BSL2) laboratory.
Main applications: Preparation of live cell samples for microscopy.

Photo: UHH/Feuerböther
M125C stereo microscope
Description: The M125C is a small stereo microscope from Leica with LED white light source. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Inspection and preparation of microscopy specimens.

Photo: UHH/Feuerböther
Analysis computer
Description: The analysis computer has 768 GB of memory and two NVIDIA RTX A6000 graphics cards for processing and analyzing large amounts of image data. FIJI, Imaris, Arivis and Matlab image analysis software are available on the computer.
Main applications: Image processing and quantitative image analysis of microscopy data.

Photo: UHH/Feuerböther
SP8 confocal microscope with FALCON FLIM unit
Description: This inverted point-scanning confocal microscope from Leica is equipped with a 405 nm laser, a white light laser and 4 hybrid detectors. The microscope has an incubation unit for live cell imaging and a FALCON FLIM unit. The microscope is programmable via the Navigator module of the Leica LASX software. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Confocal microscopy, FLIM, FRET, live cell microscopy.

Photo: UHH/Feuerböther
cryoCLEM widefield microscope
Description: This upright widefield microscope from Leica is equipped with a cryo-stage for imaging vitrified specimens on electron microscopy grids under constant cooling with liquid nitrogen. A mercury metal halide burner (EL6000) is available as the fluorescence excitation light source. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Light cryo-microscopy, widefield microscopy, correlative light- and electron-cryo-microscopy.

Photo: UHH/Feuerböther
cryoCLEM Stellaris 8 confocal microscope
Description: This upright confocal microscope from Leica is equipped with a cryo-stage for imaging vitrified specimens on electron microscopy grids under constant cooling with liquid nitrogen. Available fluorescence excitation light sources include a 405 nm pulsed laser, a white light pulsed laser, and 488 nm, 561 nm, and 633 nm cw lasers. The microscope is equipped with four hybrid detectors and has a FLIM unit. In addition, the microscope can be operated in widefield mode and has LED a fluorescence widefield excitation light source. The microscope is programmable via the Navigator module of the Leica LASX software. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Light cryo-microscopy, confocal microscopy, FLIM, FRET, widefield microscopy, correlative light- and electron-cryo-microscopy.

Photo: UHH/Feuerböther
DMi8 inverted widefield microscope for live cell imaging and with Primo Alveole micropatterning unit
Description: This inverted widefield microscope from Leica is equipped with an incubation unit for live cell measurements and an LED fluorescence excitation light source. In addition, a Primo Alveole micropatterning unit is attached. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Live cell microscopy, widefield microscopy, phase contrast microscopy, DIC, micropatterning.

Photo: UHH/Feuerböther
DMi8 inverted widefield microscope for live cell imaging and THUNDER Software
Description: This inverted widefield microscope from Leica is equipped with an incubation unit for live cell measurements, an LED fluorescence excitation light source, and THUNDER software for computational clearing. The microscope is programmable for complex tasks, such as scanning multiwell plates. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Live cell microscopy, widefield microscopy, phase contrast microscopy, DIC, scanning of multiwell plates.

Photo: UHH/Feuerböther
DM6 B upright widefield microscope #1
Description: This upright widefield microscope from Leica is equipped with a fluorescence excitation light source. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Widefield microscopy, phase contrast microscopy, DIC, histology.

Photo: UHH/Feuerböther
DM6 B upright widefield microscope #2
Description: This upright widefield microscope from Leica is equipped with a fluorescence excitation light source and is fully motorized. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Widefield microscopy, phase contrast microscopy, DIC, histology.

Photo: UHH/Feuerböther
DM6 B upright widefield microscope #3
Description: This upright widefield microscope from Leica is equipped with a fluorescence excitation light source. The microscope operated in the BSL2 lab of Tim Gilberger at CSSB Hamburg.
Main applications: Widefield microscopy, phase contrast microscopy, DIC, histology.

Photo: UHH/Feuerböther
SR GSD 3D Super-resolution microscope
Description: This inverted microscope from Leica is equipped for Single Molecule Localization Microscopy (SMLM) super-resolution microscopy and a special stage which is directly coupled to the objective. Fluorescence excitation can be performed in widefield mode or in Total Internal Reflection Fluorescence (TIRF) mode. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Super-resolution microscopy, TIRF, STORM, DNA-PAINT.

Photo: UHH/Feuerböther
Spinning-Disc and SIM microscope with FRAP unit
Description: This inverted microscope from Nikon has multiple modes in which it can be operated. There is a Yokogawa CSU W1 spinning disc unit with an Andor iXon EMCCD camera and a Nikon E-SIM unit for Structured Illumination Microscopy (SIM). In addition, the microscope is equipped with a Fluorescence Recovery after Photobleaching (FRAP) unit from Rapp Optoelectronics. An incubation unit for live cell measurements is available. The microscope is fully programmable via the JOBS module in Nikon NIS Elements software. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Spinning disc confocal microscopy, SIM, FRAP, photoactivation, live cell microscopy, scanning of multiwell plates.

Photo: UHH/Feuerböther
LSM880 Airyscan confocal microscope
Description: This inverted point-scanning confocal microscope from Zeiss has an Airyscan detector, which can be used to achieve an approximately 1.8-fold increase in resolution over normal confocal microscopes, and a spectral Imaging detector. It is equipped with an incubation unit for live cell measurements. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Confocal microscopy, live cell microscopy, airyscan microscopy.

Photo: UHH/Feuerböther
Elyra PS.1 Super-resolution microscope
Description: This inverted super-resolution microscope from Zeiss can be used for Single Molecule Localization Microscopy (SMLM) microscopy and Structured Illumination Microscopy (SIM). Fluorescence excitation can be performed in widefield mode or in Total Internal Reflection Fluorescence (TIRF) mode. It is equipped with an incubation chamber for live cell measurements. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Super-resolution microscopy, TIRF, STORM, PALM, DNA-PAINT, SIM, wide-field microscopy.

Photo: UHH/Feuerböther
Lattice light sheet microscope
Description: This lattice light sheet microscope from Zeiss is suitable for fast volumetric imaging of living cells with particularly low phototoxicity and simultaneously high local resolution. It features two Hamamatsu sCMOS cameras. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Lattice light sheet microscopy, live cell microscopy.

Photo: UHH/Feuerböther
Cell culture laminar flow work bench and incubators
Description: The cell culture laminar flow work bench and two incubators are fully equipped to allow ALFM Facility users to prepare and pre-incubate their live cell samples before microscopy. The cell culture workbench and the incubators are located in a biological safety level 2 (BSL2) laboratory.
Main applications: Preparation of live cell samples for microscopy.

Photo: UHH/Feuerböther
M125C stereo microscope
Description: The M125C is a small stereo microscope from Leica with LED white light source. The microscope is operated in a biological safety level 2 (BSL2) laboratory.
Main applications: Inspection and preparation of microscopy specimens.

Photo: UHH/Feuerböther
Analysis computer
Description: The analysis computer has 768 GB of memory and two NVIDIA RTX A6000 graphics cards for processing and analyzing large amounts of image data. FIJI, Imaris, Arivis and Matlab image analysis software are available on the computer.
Main applications: Image processing and quantitative image analysis of microscopy data.
